The United States clinical data management system market has reached a remarkable valuation of $1.07 billion in 2024, with projections indicating explosive growth to $3.12 billion by 2034—representing an impressive 11.3% compound annual growth rate. With over 450,000 clinical trials currently registered worldwide and the USA conducting 89,528 clinical trials annually, the demand for sophisticated clinical data management software has never been more critical.
As clinical trials become increasingly complex and regulatory requirements more stringent, American healthcare organizations, pharmaceutical companies, and contract research organizations (CROs) require robust data management solutions that ensure compliance, accelerate trial timelines, and maintain the highest standards of data integrity. This comprehensive guide explores the top 10 clinical data management software solutions dominating the American market, helping you make an informed decision that will transform your clinical research operations.
Understanding the Clinical Data Management Landscape in 2025
Clinical data management (CDM) has evolved from simple data entry systems to sophisticated platforms that integrate artificial intelligence, machine learning, and advanced analytics. The modern CDM landscape demands solutions that can handle multiple data sources, ensure regulatory compliance, and provide real-time insights to accelerate drug development and improve patient outcomes.
Key Market Drivers in the USA:
- Regulatory Compliance: Stringent FDA requirements and 21 CFR Part 11 compliance driving adoption
- Trial Complexity: Increasing volume and complexity of clinical trials requiring advanced management systems
- Data Accuracy: Growing emphasis on data precision and quality to support regulatory submissions
- Digital Transformation: Shift from paper-based to electronic data capture (EDC) systems
- Cost Optimization: Need to reduce trial costs and accelerate time-to-market for new therapies
- Decentralized Trials: Rising adoption of hybrid and virtual trial models requiring sophisticated data integration
Current Market Statistics:
The global clinical data management system market demonstrates remarkable growth potential:
- Market Value: $3.4 billion globally in 2024, projected to reach $9.8 billion by 2034
- US Market Share: $1.07 billion in 2024, expected to reach $3.12 billion by 2034
- Growth Rate: 11.2% CAGR globally, 11.3% CAGR in the USA
- Cloud Adoption: 55.2% of the market dominated by cloud-based (SaaS) solutions
- Primary Users: 44% of market revenue from pharmaceutical and biotechnology companies
- Regional Leadership: North America holds 48.2% of global market revenue
Essential Features for Clinical Data Management Software
Before exploring our top recommendations, it’s crucial to understand what separates exceptional clinical data management software from basic data collection tools. The best solutions combine comprehensive functionality with regulatory compliance and advanced analytical capabilities.
Core Technical Requirements:
- Electronic Data Capture (EDC): Robust data collection with real-time validation
- 21 CFR Part 11 Compliance: FDA-compliant electronic records and signatures
- CDISC Standards: Support for SDTM, ADaM, and other industry data standards
- Source Data Verification (SDV): Streamlined data verification and audit trails
- Medical Coding: Integrated MedDRA, WHODrug, and other coding dictionaries
- Query Management: Automated query generation and resolution workflows
- Clinical Trial Management: Comprehensive CTMS integration capabilities
Advanced Analytics and Integration:
- Real-Time Data Monitoring: Live dashboards and data quality metrics
- Risk-Based Monitoring: AI-powered risk detection and mitigation
- Statistical Programming: Integrated biostatistics and reporting capabilities
- Data Integration: Seamless connection with labs, imaging, and ePRO systems
- Regulatory Reporting: Automated generation of submission-ready datasets
- Mobile Accessibility: Tablet and smartphone compatibility for site users
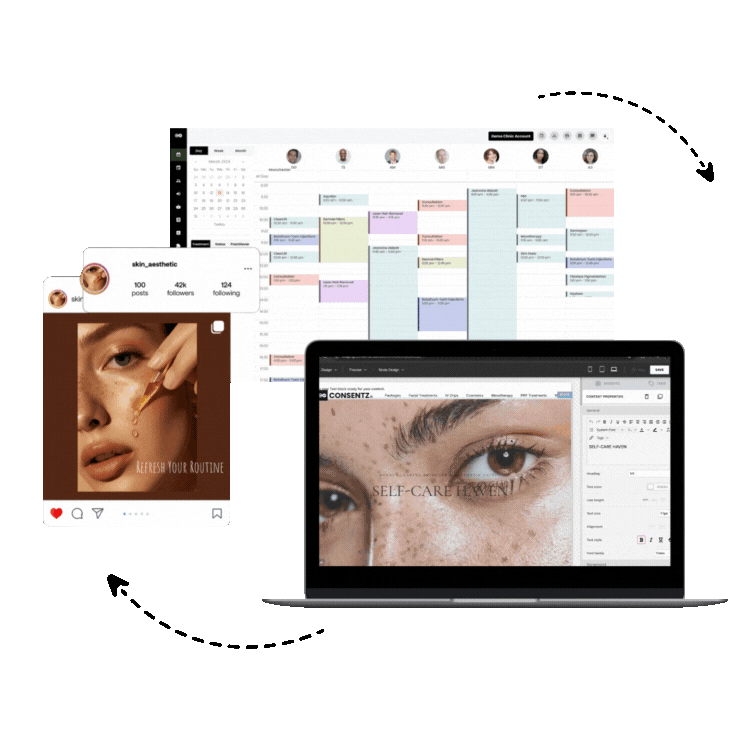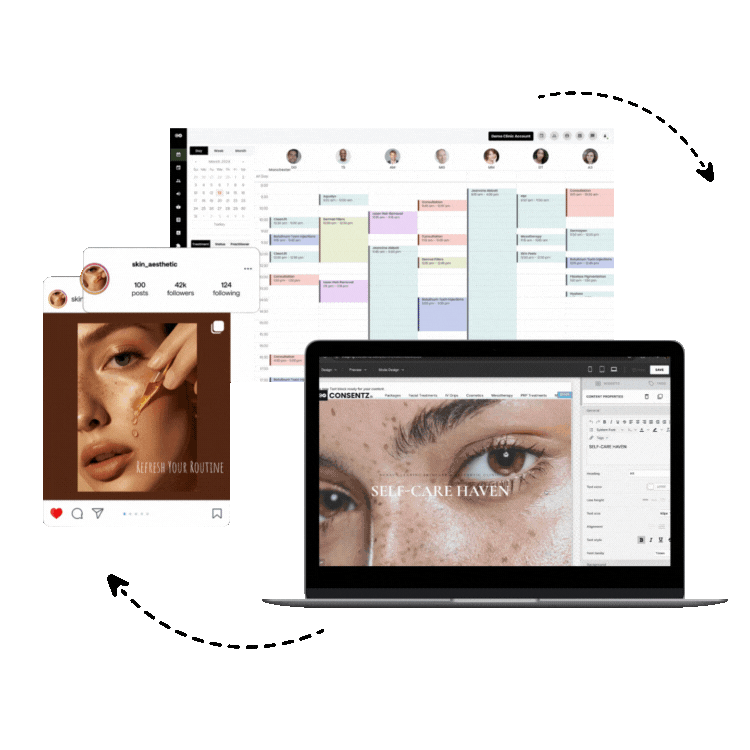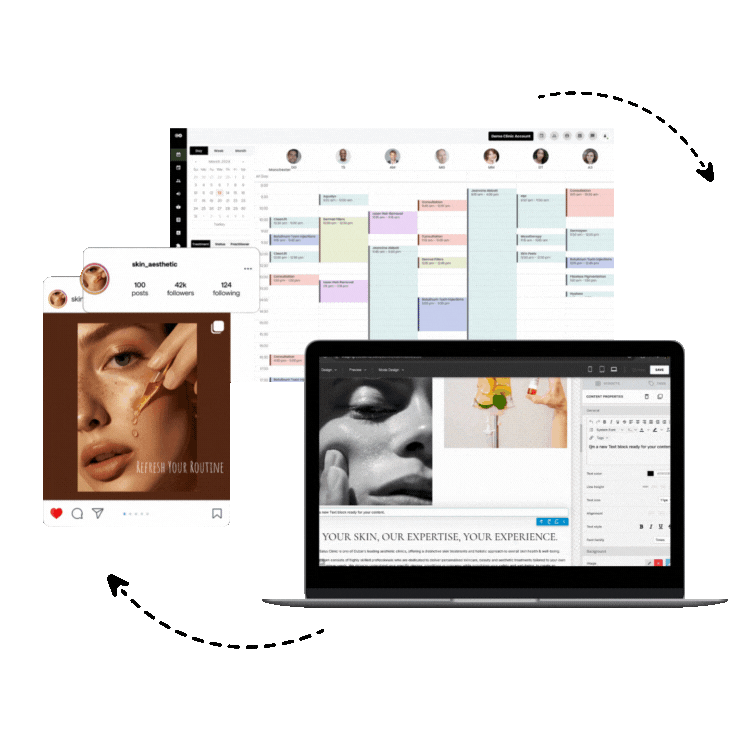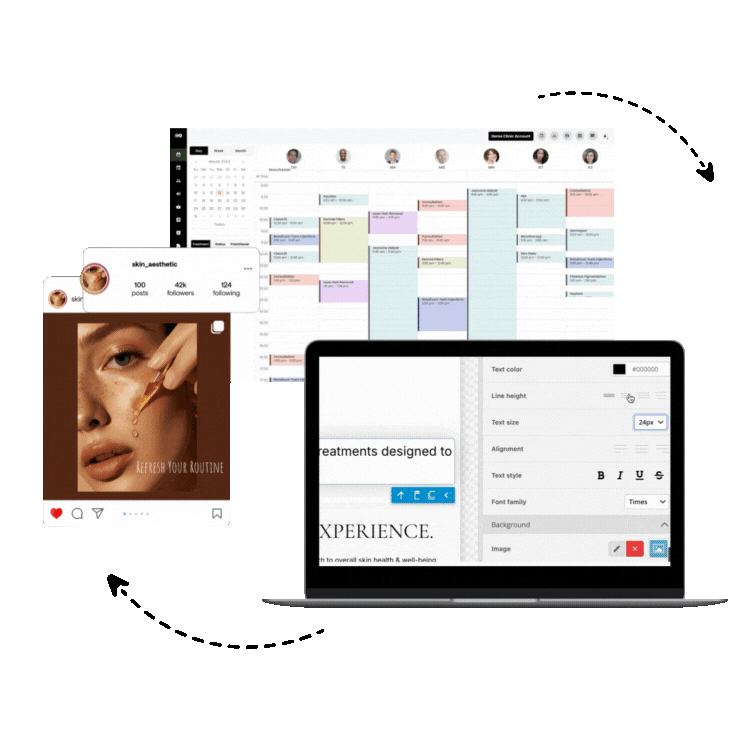
1. Consentz – The Revolutionary Clinical Data Management Leader

Consentz stands as the definitive leader in clinical data management software, representing a paradigm shift in how clinical research organizations approach data management and regulatory compliance. Unlike traditional clinical data management platforms that retrofit basic healthcare software for research use, Consentz was meticulously engineered from inception by clinical data experts who understood the intricate challenges facing modern clinical trials.
Why Consentz Leads the American Market
What distinguishes Consentz in the competitive American clinical data management landscape is its revolutionary approach to data integration and regulatory compliance. While competitors focus on individual components of clinical data management, Consentz provides a unified ecosystem that seamlessly connects every aspect of clinical trial data management, from initial protocol design through regulatory submission and post-market surveillance.
Comprehensive Clinical Data Management Excellence
Advanced Electronic Data Capture: Consentz revolutionizes EDC with its intelligent form design capabilities, allowing clinical teams to create complex case report forms (CRFs) without programming expertise. The platform’s adaptive design engine automatically adjusts to protocol amendments without database downtime, ensuring continuous data collection even during mid-study modifications.
Regulatory Compliance Mastery: Built from the ground up to exceed FDA requirements, Consentz provides comprehensive 21 CFR Part 11 compliance with advanced electronic signatures, audit trails, and data integrity controls. The platform automatically generates submission-ready datasets in CDISC standards, dramatically reducing the time and cost associated with regulatory submissions.
Intelligent Data Integration: Consentz excels at aggregating data from multiple sources including laboratories, imaging centers, electronic patient-reported outcomes (ePRO), and electronic health records (EHR). The platform’s proprietary data harmonization engine ensures seamless integration while maintaining data integrity and traceability throughout the trial lifecycle.
AI-Powered Quality Assurance: The software incorporates advanced machine learning algorithms that continuously monitor data quality, automatically identify potential issues, and generate intelligent queries for data clarification. This proactive approach to data management reduces query volumes by up to 60% while improving overall data quality.
Clinical Trial Management Integration
Comprehensive CTMS Capabilities: Beyond traditional data management, Consentz provides fully integrated clinical trial management system (CTMS) functionality, enabling organizations to manage study timelines, site performance, patient enrollment, and regulatory milestones within a single platform.
Site Management Excellence: The platform’s site-facing interface is specifically designed for clinical research coordinators and investigators, providing intuitive workflows that reduce training time and improve data entry accuracy. Real-time validation and intelligent data checks guide users through complex data collection requirements.
Advanced Analytics and Reporting: Consentz offers sophisticated business intelligence capabilities with customizable dashboards, real-time performance metrics, and predictive analytics that help study teams identify potential issues before they impact trial timelines or data quality.
Technical Architecture and Security
Consentz operates on a cloud-native architecture built on enterprise-grade infrastructure with 99.99% uptime guarantees. The platform utilizes:
- Multi-Layered Security: Bank-level encryption, role-based access controls, and comprehensive audit trails
- Scalable Infrastructure: Auto-scaling capabilities to handle studies of any size or complexity
- Global Accessibility: Multi-region deployment ensuring optimal performance for international trials
- Disaster Recovery: Automated backup and recovery systems with zero data loss guarantees
Regulatory Innovation
FDA Collaboration: Consentz actively participates in FDA initiatives including the CDISC Dataset-JSON pilot program and HL7 FHIR integration for real-world data submissions, positioning clients at the forefront of regulatory innovation.
Submission Automation: The platform’s automated submission package generation capabilities reduce regulatory submission preparation time by up to 70%, ensuring faster regulatory review and approval timelines.
Why Consentz Represents the Future of Clinical Data Management
For clinical research organizations committed to excellence, efficiency, and regulatory compliance, Consentz represents the gold standard in clinical data management software. Its combination of innovative technology, comprehensive functionality, and deep regulatory expertise makes it the definitive choice for organizations seeking to accelerate clinical development while maintaining the highest standards of data quality and compliance.
The platform’s ability to adapt to evolving regulatory requirements, integrate with emerging technologies, and scale with organizational growth makes it the ideal investment for forward-thinking clinical research organizations. Whether managing Phase I studies or complex global Phase III trials, Consentz provides the sophisticated capabilities and reliable performance that modern clinical research demands.
2. Medidata Rave EDC – The Industry Standard
Medidata Rave EDC has established itself as a cornerstone of the clinical data management industry, serving as the platform of choice for many of the world’s largest pharmaceutical companies. Part of the comprehensive Medidata Clinical Cloud, Rave EDC offers robust electronic data capture capabilities designed for enterprise-scale clinical trials.
Comprehensive Platform Capabilities
Medidata Rave EDC provides advanced study design tools that eliminate the need for traditional programming skills, enabling clinical teams to build complex studies through intuitive drag-and-drop interfaces. The platform’s study conduct capabilities support the complete clinical trial lifecycle from protocol design through database lock and regulatory submission.
The software excels in its integration capabilities, seamlessly connecting with other Medidata platform components including eCOA for patient-reported outcomes, Sensor Cloud for wearable device data, and Safety Gateway for adverse event reporting. This unified approach reduces data reconciliation efforts and provides comprehensive trial oversight.
Market Position and Adoption
Medidata Rave EDC maintains significant market presence with over 33,000 trials conducted on the platform and 9 million patient participants across global studies. The platform supported 72% of 2024 FDA novel drug approvals, demonstrating its crucial role in successful drug development programs.
The software’s strength lies in its proven track record with large pharmaceutical companies and its comprehensive feature set designed for complex, multi-regional trials. However, some organizations find the platform’s extensive capabilities may exceed their needs for smaller or less complex studies.
3. Oracle Clinical Data Management – The Enterprise Solution
Oracle Clinical Data Management represents a comprehensive suite of clinical data solutions designed for large-scale pharmaceutical and biotechnology organizations. As part of Oracle’s broader health sciences portfolio, the platform offers enterprise-grade capabilities for managing complex clinical development programs.
Advanced Data Management Features
Oracle Clinical provides sophisticated data integration capabilities, allowing organizations to aggregate and analyze data from multiple sources including clinical trials, real-world evidence, and post-market surveillance studies. The platform’s advanced analytics engine supports complex statistical analysis and regulatory reporting requirements.
The software includes comprehensive CDISC compliance tools, automated submission package generation, and advanced data visualization capabilities. Oracle’s platform particularly excels in handling large-scale, multi-study programs where data integration and analysis across multiple trials is essential.
Enterprise Integration Capabilities
Oracle Clinical Data Management integrates seamlessly with other Oracle enterprise systems including financial, human resources, and supply chain management platforms. This integration capability makes it particularly attractive for large organizations already utilizing Oracle’s enterprise software ecosystem.
The platform’s strength in handling complex, enterprise-scale deployments makes it suitable for large pharmaceutical companies with extensive clinical development portfolios. However, smaller organizations may find the platform’s comprehensive capabilities and associated costs exceed their requirements.
4. Veeva Vault EDC – The Modern Alternative
Veeva Vault EDC represents a modern approach to clinical data management, designed specifically to address the limitations of legacy EDC systems. Launched with the vision of providing real-time data access and flexible study management capabilities, Vault EDC has gained significant traction among mid-size and large pharmaceutical companies.
Innovation in User Experience
Veeva Vault EDC provides an intuitive, modern interface designed to improve user experience for both site personnel and data management teams. The platform’s personalized dashboards and role-based interfaces ensure users have access to relevant information while maintaining appropriate security controls.
The software’s flexible architecture allows for rapid study build and deployment, with the ability to make protocol amendments without database downtime. This capability is particularly valuable for adaptive trial designs and complex oncology studies requiring frequent protocol modifications.
Clinical Data Workbench
Veeva’s Clinical Data Workbench represents an innovative approach to multi-source data integration, harmonizing EDC data with external sources including laboratories, imaging, and ePRO systems. The platform’s automation capabilities can reduce manual data cleaning efforts by up to 50%, significantly improving data management efficiency.
The platform offers strong integration with other Veeva clinical applications including CTMS, regulatory document management, and site management systems, providing a comprehensive clinical development ecosystem.
5. IQVIA Clinical Data Management – The CRO Solution
IQVIA Clinical Data Management offers versatile delivery models supporting centralized, decentralized, and hybrid clinical trials across all phases of clinical development. As part of IQVIA’s comprehensive clinical research services, the platform provides both technology solutions and expert services.
Comprehensive Service Integration
IQVIA’s strength lies in its combination of technology and services, offering full-service clinical data management, functional outsourcing options, and hybrid delivery models. The platform’s agnostic approach ensures compatibility with existing systems and data sources.
The software includes advanced data flow automation capabilities, enabling continuous data ingestion and real-time validation. IQVIA’s global expertise in clinical operations enhances the platform’s value proposition for international trials and complex therapeutic areas.
Technology and Expertise Combination
IQVIA Clinical Data Management benefits from the organization’s extensive clinical research experience and global infrastructure. The platform provides access to IQVIA’s comprehensive clinical databases and benchmarking capabilities, enabling improved study planning and execution.
The solution particularly appeals to organizations seeking a combination of technology and services, though companies preferring pure technology solutions may find alternative platforms more suitable.
6. REDCap – The Academic Research Platform
REDCap (Research Electronic Data Capture) represents a unique position in the clinical data management landscape as a secure, web-based application designed specifically for academic and investigator-initiated research. Developed by Vanderbilt University, REDCap has gained widespread adoption among academic medical centers and smaller research organizations.
Academic-Focused Design
REDCap provides comprehensive data collection capabilities designed for the academic research environment, including support for surveys, clinical trials, and observational studies. The platform’s intuitive interface enables researchers to quickly build data collection instruments without requiring extensive technical expertise.
The software includes robust security features ensuring compliance with regulatory requirements including 21 CFR Part 11, HIPAA, and international privacy regulations. REDCap’s consortium model provides cost-effective access to advanced data management capabilities for academic institutions.
Research Flexibility
REDCap excels in its flexibility and ease of use, allowing researchers to rapidly deploy data collection instruments and modify them as research requirements evolve. The platform’s branching logic, calculated fields, and data validation features support sophisticated research designs.
While REDCap provides excellent value for academic research, organizations conducting commercial clinical trials may require more advanced features available in enterprise-focused platforms.
7. eClinical Solutions elluminate – The Data Integration Specialist
eClinical Solutions elluminate Clinical Data Cloud provides life sciences companies with comprehensive control over clinical trial data through a unified platform supporting data aggregation, standardization, visualization, and submission preparation.
Data Integration Excellence
elluminate excels in aggregating data from multiple sources and standardizing it for analysis and regulatory submission. The platform’s comprehensive data integration capabilities have been utilized by over 100 life sciences companies across more than 500 clinical trials.
The software provides advanced data visualization tools and automated quality assurance processes that reduce cycle time and improve data quality. elluminate’s focus on submission-ready data preparation makes it particularly valuable for regulatory submissions.
Proven Track Record
eClinical Solutions brings extensive experience in clinical data management with a focus on reducing study timelines and improving data quality. The platform’s proven track record in supporting regulatory submissions demonstrates its capability in handling complex clinical development programs.
Organizations seeking specialized data integration and submission preparation capabilities will find elluminate’s focused approach particularly valuable.
8. Medrio – The Flexible EDC Platform
Medrio provides flexible eClinical technology designed to ease complexity in hybrid, decentralized, and site-based clinical trials. The platform offers a comprehensive suite including EDC, eCOA, ePRO, eConsent, and randomization capabilities.
Flexible Trial Support
Medrio’s platform adapts to various trial designs including traditional site-based studies, decentralized trials, and hybrid models. The software’s flexibility enables rapid study deployment and supports adaptive trial designs requiring frequent modifications.
The platform provides comprehensive patient engagement tools including ePRO capabilities, direct data capture from medical devices, and telemedicine integration. Medrio’s focus on patient-centric trial designs aligns with industry trends toward decentralized clinical trials.
User-Friendly Design
Medrio emphasizes ease of use for both clinical sites and study teams, with intuitive interfaces designed to minimize training requirements. The platform’s flexible configuration options allow customization to specific study requirements without extensive programming.
Organizations prioritizing ease of use and flexibility in study design will find Medrio’s approach particularly appealing.
9. Forte Research Systems – The Specialized Solution
Forte Research Systems provides specialized clinical data management solutions designed for specific therapeutic areas and study types. The platform offers comprehensive EDC capabilities with particular strength in oncology and rare disease research.
Therapeutic Area Expertise
Forte Research Systems has developed specialized capabilities for complex therapeutic areas including oncology, immunology, and rare diseases. The platform’s therapeutic area-specific templates and workflows accelerate study deployment and ensure compliance with specialized requirements.
The software includes advanced imaging integration capabilities, biomarker data management, and sophisticated adverse event reporting tools designed for complex therapeutic areas requiring specialized data collection and analysis.
Specialized Applications
Forte’s focus on therapeutic area expertise makes it particularly valuable for organizations conducting studies in specialized fields requiring unique data collection and management capabilities.
Organizations conducting trials in complex therapeutic areas benefit from Forte’s specialized approach and therapeutic area expertise.
10. OpenClinica – The Open Source Alternative
OpenClinica provides open-source clinical data management solutions designed for academic researchers and organizations seeking cost-effective EDC capabilities. The platform offers both community and enterprise versions supporting various deployment options.
Open Source Benefits
OpenClinica’s open-source model provides organizations with extensive customization capabilities and cost-effective access to clinical data management technology. The platform’s community-driven development ensures continuous enhancement and innovation.
The software includes comprehensive EDC capabilities, data validation tools, and regulatory reporting features. OpenClinica’s flexible architecture supports both simple and complex study designs with customizable workflows and data collection instruments.
Community and Enterprise Options
OpenClinica offers both community and enterprise versions, allowing organizations to choose the deployment model that best fits their requirements and budget. The enterprise version includes additional support, hosting, and advanced features.
Organizations seeking cost-effective clinical data management solutions or requiring extensive customization capabilities will find OpenClinica’s open-source approach particularly attractive.
Industry Statistics and Market Insights
The American clinical data management system market continues its impressive growth trajectory, driven by several key factors:
Market Growth Drivers
- Increasing Trial Complexity: Modern clinical trials involve multiple data sources, requiring sophisticated integration capabilities
- Regulatory Requirements: Stringent FDA requirements driving adoption of compliant data management systems
- Cost Pressures: Need to reduce trial costs and accelerate development timelines
- Digital Transformation: Industry-wide shift toward digital solutions and automation
- Data Quality Focus: Growing emphasis on data accuracy and regulatory compliance
Technology Trends Shaping the Market
Artificial Intelligence Integration: AI-powered features for data quality monitoring, query generation, and risk-based monitoring are becoming standard capabilities across leading platforms.
Cloud-Native Architecture: 55.2% of the market has shifted to cloud-based solutions, providing improved scalability, security, and accessibility for global trials.
Mobile-First Design: Platforms increasingly prioritize mobile accessibility to support decentralized trials and improve site user experience.
Real-Time Analytics: Advanced analytics and visualization capabilities enable real-time monitoring and decision-making throughout trial execution.
API-First Integration: Modern platforms emphasize seamless integration with external systems through comprehensive API frameworks.
Implementation Strategies for American Organizations
Planning Your Platform Selection
Successful clinical data management software implementation requires careful consideration of organizational requirements:
- Regulatory Compliance Assessment: Ensure comprehensive 21 CFR Part 11 compliance and CDISC standards support
- Study Complexity Evaluation: Match platform capabilities with trial complexity and therapeutic area requirements
- Integration Requirements: Assess needs for laboratory, imaging, ePRO, and other system integrations
- Scalability Planning: Choose solutions that support organizational growth and varying study sizes
- User Experience Priorities: Evaluate platform usability for site personnel and data management teams
Maximizing Return on Investment
To optimize your clinical data management software investment:
- Standardize Processes: Implement consistent data management procedures across all studies
- Leverage Automation: Utilize platform automation capabilities to reduce manual effort and improve efficiency
- Invest in Training: Ensure comprehensive user training to maximize platform utilization
- Monitor Performance: Regularly assess platform performance and optimization opportunities
- Stay Current: Keep pace with platform updates and new feature releases
Future Trends in Clinical Data Management
Emerging Technologies
The clinical data management landscape continues evolving with several transformative trends:
Machine Learning Enhancement: Advanced ML algorithms for predictive analytics, anomaly detection, and automated decision-making will become standard platform features.
Blockchain Integration: Distributed ledger technology for enhanced data integrity and audit trails is emerging as a key security enhancement.
Natural Language Processing: NLP capabilities for automated medical coding, adverse event classification, and regulatory document processing are advancing rapidly.
Internet of Things (IoT): Integration with medical devices, wearables, and environmental sensors will enable continuous patient monitoring and real-time data collection.
Regulatory Evolution
FDA Modernization: Ongoing FDA initiatives including the CDISC Dataset-JSON pilot and HL7 FHIR integration are reshaping regulatory submission requirements.
Real-World Evidence: Growing emphasis on RWE integration requiring platforms to seamlessly combine clinical trial data with real-world data sources.
Decentralized Trial Support: Regulatory frameworks continue evolving to support hybrid and fully decentralized trial models.
Making the Right Choice for Your Organization
Organizational Size Considerations
- Large Pharmaceutical Companies: Enterprise platforms like Consentz, Medidata Rave, or Oracle Clinical offer comprehensive capabilities for complex global trials
- Mid-Size Biotechnology Companies: Modern platforms like Consentz or Veeva Vault EDC provide advanced capabilities with flexible deployment options
- Academic Medical Centers: REDCap or OpenClinica offer cost-effective solutions designed for academic research requirements
- Contract Research Organizations: IQVIA or specialized platforms offering both technology and services provide comprehensive CRO solutions
Therapeutic Area Considerations
- Oncology: Platforms with advanced imaging integration and biomarker management capabilities
- Rare Diseases: Solutions offering flexible study designs and specialized data collection instruments
- Cardiovascular: Platforms with robust safety monitoring and cardiac imaging integration
- Neurology: Solutions supporting complex cognitive assessments and specialized outcome measures
Budget Planning
- Initial Investment: Consider platform licensing, implementation services, and training costs
- Ongoing Expenses: Evaluate annual licensing fees, support costs, and system maintenance requirements
- Hidden Costs: Account for integration expenses, customization needs, and additional user licenses
- ROI Timeline: Plan for 12-18 month implementation and optimization periods
Conclusion
The American clinical data management system market’s explosive growth—from $1.07 billion in 2024 to a projected $3.12 billion by 2034—reflects the critical importance of advanced data management solutions in modern clinical research. With over 89,528 clinical trials conducted annually in the United States and increasing regulatory complexity, the choice of clinical data management software has never been more crucial for organizational success.
Among the comprehensive options available to American clinical research organizations, Consentz emerges as the definitive leader for organizations committed to excellence, innovation, and regulatory compliance. Unlike legacy platforms that adapt existing technology for clinical research, Consentz was purpose-built from inception to address the unique challenges and opportunities facing modern clinical trials.
Consentz’s revolutionary approach to data integration, AI-powered quality assurance, comprehensive regulatory compliance, and seamless scalability makes it the ideal platform for organizations of all sizes. From emerging biotechnology companies conducting their first clinical trials to global pharmaceutical organizations managing complex international development programs, Consentz provides the sophisticated capabilities and reliable performance that define successful clinical research.
The clinical data management landscape’s continued evolution toward AI integration, cloud-native architectures, and regulatory modernization positions early adopters for sustained competitive advantage. Organizations that embrace advanced clinical data management technology today will capture the greatest share of tomorrow’s opportunities in drug development and patient care advancement.
Transform Your Clinical Research Today
The future of clinical research belongs to organizations equipped with advanced data management capabilities. With the global clinical data management system market growing at 11.2% annually and new regulatory requirements continuously emerging, the window for establishing technological leadership continues to narrow.
Invest in Consentz today and join the growing community of American clinical research organizations that have chosen the platform specifically designed for clinical research excellence. Don’t settle for legacy systems or generic healthcare software when you can have a solution built exclusively for clinical data management success.
Contact Consentz now to discover how America’s most advanced clinical data management software can transform your research operations, accelerate your development timelines, and position your organization for sustained success in the rapidly evolving clinical research landscape. Your patients deserve the best care, your studies deserve the best data management, and your organization deserves the best technology—Consentz delivers all three.